Research
- Essential roles of synaptic transmission in brain functions
- Understanding molecular basis of synaptic function
- New properties of synapses revealed by imaging approaches
- Synapses are continuously built and eliminated during development
- Postsynaptic scaffolding molecules are densely packed within the PSD but dynamic
- Astrocytic contacts promote synaptogenesis
- Diversity in synapse formation
- Application of synapse imaging in studies of mental disorders
- Future directions in the research of synapse cell biology
- Building a quantitative model of synaptic molecular assembly
- Behavior of synapses in vivo
I Essential roles of synaptic transmission in brain functions
Brain functions, which are dependent on mutual communication of a tremendous number of neuronal cells, regulate the behavior of animals and humans. The main structure specifically differentiated for information exchange between neurons is called "synapse". Long-term maintenance of synaptic properties underlies stability and reproducibility of behavior in responses to external stimuli. In turn, alterations of synaptic properties are thought to be the basis of behavioral change in the course of animal development and also after learning. Therefore, synapses should be stabilized for a long term to realize fidelity of various animal behaviors, but also should be altered rapidly when animals adapt to a new environment. Molecular basis of this dichotomy, which is unique to synapses, is the main interest of our laboratory.
II Understanding molecular basis of synaptic function
Imaging is very powerful in characterizing synaptic structure and function. Synapse has been studied by using biochemical techniques, which isolate "synaptosomes" from the brain and identify individual constituents, or by electrophysiological techniques, which detect electrical signals derived from ionic currents through activated ion channels and receptors in living neurons. These two techniques have been extensively applied to the study of synapses and successful in characterizing population properties of synapses, but neither of them can provide information derived from individual synapses. An obvious advantage of optical methods is that they can detect and analyze signals from single synapses repeatedly. Therefore application of advanced optical techniques, including high sensitivity imaging of fluorescent proteins, confocal laser microscopy and multiphoton excitation of chromophores, is essential in the modern synapse research.
III New properties of synapses revealed by imaging approaches
What are the novel properties of synapses revealed by imaging approaches? Here I will summarize several new findings from our laboratory.
1. Synapses are continuously built and eliminated during development
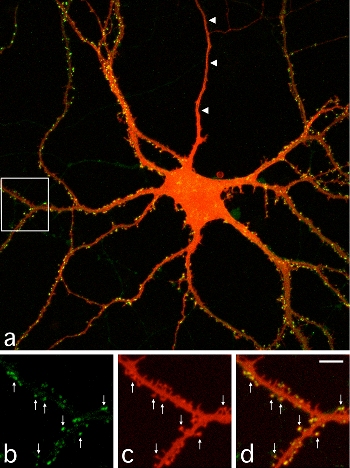 |
| III-1 A cultured hippocampal neuron expressing PSD-95 tagged with GFP. The image shows intracellular distribution of PSD-95 (Green) and neuronal morphology detected by DiI (Red). Images b-d (magnified images of the white box area in a) show spines containing PSD-95 clusters (arrows). |
It has been widely accepted that synapses are stabilized after their initial establishment and show little change thereafter. Synapse formation and elimination are thought to take place in two distinct phases of neural network development. Synapse formation is the earlier event and generates redundant connections between neurons. Synapse elimination follows and unnecessary connections are removed from the network, probably by the extrinsic instructive signals such as sensory inputs. To see if the period of synapse formation is distinct from synapse elimination, we measured lifetime of individual synapses by using GFP-tagged scaffolding proteins localized in the postsynaptic density (PSD). In cultured hippocampal neurons, synapse density monotonously increases from 1 to 3 weeks after plating. Time-lapse imaging of GFP-labeled PSDs revealed elimination of a predominant proportion of newly formed synapses even in the early period of monotonous synapse increase. Within one day, 10-20% of total synapses were eliminated and a roughly equal number of synapses were newly generated to counterbalance the elimination. Detailed analysis of the balance between synapse elimination and formation indicated a slight bias toward synapse formation, which underlies gradual increase of the total number of synapses. This observation clearly illustrates the importance of single synapse analysis to characterize the mechanism of establishing neuronal connectivity in the developing brain.
Information processing is the major function of the nervous system. Synaptic transmission plays a key role in this process and is mediated by release of neurotransmitters from the presynaptic sites, a specialized compartment of the axon, and by subsequent activation of neurotransmitter receptors clustered on the postsynaptic membrane. A major excitatory neurotransmitter in the mammalian CNS is glutamate. Glutamate receptors, such as AMPA receptors and NMDA receptors, are clustered on the postsynaptic membranes. When new synapses are formed, both presynaptic release machinery and postsynaptic receptor should be recruited. However, the time course and order of the molecular assembly at presynaptic and postsynaptic sites were not clarified. Molecular assembly and structural change of single synapses were visualized by using synaptophysin-CFP as a presynaptic marker and PSD-95-YFP as a postsynaptic marker. This imaging analysis revealed rapid formation of synaptic molecular specialization in the time scale of 30 min to several hours. Global analysis of synapse formation indicated gradual increase of synapse density, which led to the contention that formation and maturation of single synapses are also gradual. However, visualization of single synapses clearly illustrated rapid formation of single synapses, concomitant dynamic remodeling of dendrites, and associated assembly of functional molecules.
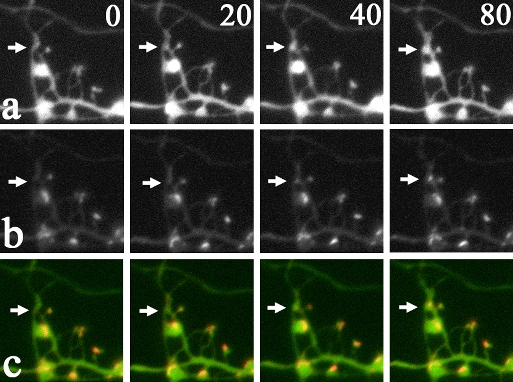 |
| III-2 Rapid formation of synaptic structure. (a) shows CFP fluorescence of dendritic and axonal structures. (b) shows PSD-95-YFP signals in assembling PSDs. (c) is the overlay of (a) and (b), indicating simultaneous formation of both spines (arrows in a) and PSDs (arrows in b). Numbers shown in (a) indicate elapsed time (min). |
- Okabe, S., Kim, H., Miwa, A., Kuriu, T., and H. Okado. Continual remodeling of postsynaptic density and its regulation by synaptic activity. Nature Neuroscience, 2, 804-811, 1999.
- Okabe, S., Miwa, A., and H. Okado. Spine formation and correlated assembly of presynaptic and postsynaptic molecules. Journal of Neuroscience, 21, 6105-6114, 2001.
- Ebihara, T., Kawabata, I., Usui, S., Sobue, K., and S. Okabe. Synchronized formation and remodeling of postsynaptic densities: long-term visualization of hippocampal neurons expressing postsynaptic density proteins tagged with GFP. Journal of Neuroscience, 23, 2170-2181, 2003.
2. Postsynaptic scaffolding molecules are dynamic and densely packed
To obtain an integrated view on both synaptic structure and dynamics of synaptic molecules, it is necessary to build a quantitative model of molecular assembly in both the PSD and the presynaptic active zone. Essential information for building such a model is the absolute numbers of molecules localized within single synapses. Conventional immunocytochemistry and immunoblotting provided data on relative amount of proteins in specific locations or preparations, but could not report the absolute content of proteins per synapse. To reveal the number of specific PSD scaffolding proteins per synapse, we developed a new quantitative technique of fluorescence microscopy. We first compared fluorescence intensities of single GFP molecules and small fluorescent microspheres and calibrated the fluorescence intensity of fluorescent microspheres. Next we obtained confocal images of single synapses expressing GFP-tagged PSD scaffolding proteins with calibrated fluorescent microspheres attached to the culture substrate, measured signals derived from two fluorescent objects, and calculated the number of GFP molecules within single synaptic sites. Finally, we performed quantitative immunocytochemistry to determine the relative level of overexpression of GFP-tagged PSD scaffolding proteins in transfected neurons. From these parameters, it is possible to estimate the absolute numbers of endogenous PSD scaffolding proteins localized within single synaptic sites. This approach enables us to obtain the absolute synaptic contents of multiple PSD scaffolding proteins (PSD-95, Homer, GKAP, and Shank molecules), which are in the range of 100 to 500 per synapse. From the quantitative data, we propose a model of molecular architecture of the PSD, which indicates relatively dense distribution of PSD-95 in the PSD structure and high probability of PSD-95 and NMDA receptor interaction by random collision of two molecules in the microenvironment of the postsynaptic membrane.
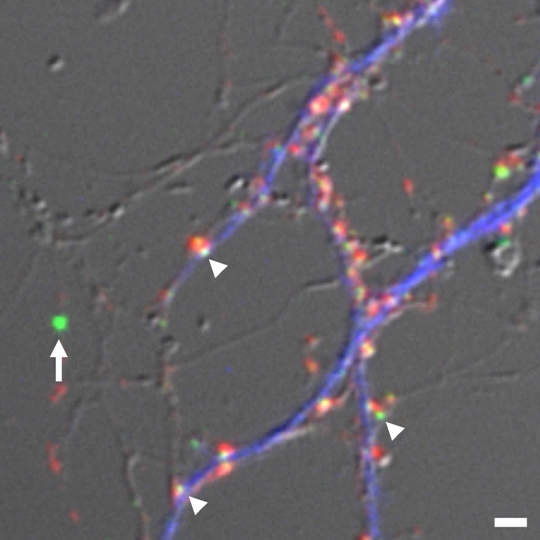 |
| III-3 Estimation of absolute numbers of GFP-tagged PSD scaffolding molecules. We determined relative fluorescence ratio of fluorescent microspheres (arrow), calibrated against single GFP molecules, and individual PSD clusters (arrowheads) and estimated absolute numbers of GFP-tagged PSD scaffolding molecules in single synapses. Presynaptic boutons (red) and dendritic shafts (blue) were also identified by synaptophysin staining and MAP2 staining, respectively. |
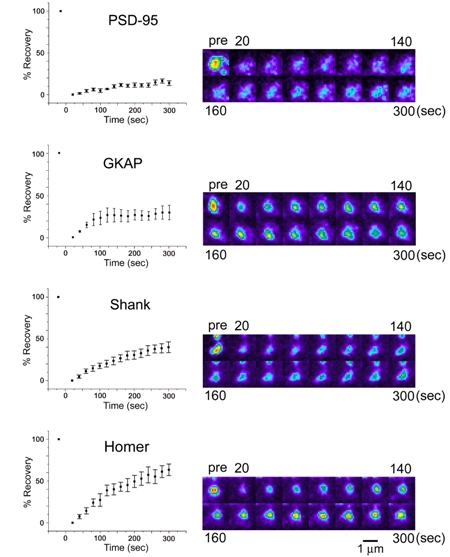 |
| III-4 Turnover rate of four PSD scaffolding molecules estimated by fluorescence recovery after photobleaching (FRAP). Left graphs show quantitation of fluorescence recovery. PSD-95, GKAP, Shank, and Homer molecules show distinct recovery profiles. |
How is assembly of individual postsynaptic scaffolding molecules regulated and how is it related to the large-scale remodeling of synapse structure? By using a technique of fluorescence recovery after photobleaching (FRAP), turnover rates of PSD scaffolding molecules were estimated at the level of single synapses. Time constants of turnover were in a range of several minutes to several tens of minutes, indicating faster assembly-disassembly rate of scaffolding molecules compared with the life time of the PSDs themselves. This observation indicates continuous replacement of synaptic molecules within the established PSDs and we can expect complete renewal of molecular constituents of the PSD if we wait only for a few hours. An important question is, in spite of the complete renewal of molecular constituents every few hours, why various parameters of synaptic functions, such as transmission efficacy, can be preserved for a long period. Currently we have no definitive answer to this question. One possibility is that we are still missing the key molecules which permanently reside within the established PSD. Another possibility is that even without persistent molecules in the PSD, molecular interactions in the synaptic cytoplasm is designed to maintain synapse stability, possibly by using feedback regulatory system. The latter possibility is more attractive, as molecular machines designed to keep their own functional states may be a fundamental component of cellular signaling systems. Our imaging analysis revealed that the actin cytoskeleton is an important regulator of postsynaptic scaffolding molecules. One obvious possibility is that bidirectional regulation between the PSD and the actin cytoskeleton is a part of the above mentioned feedback regulatory system.
- Sugiyama, Y., Kawabata, I., Sobue, K., and S. Okabe Determination of absolute protein numbers in single synapses by a GFP-based calibration technique. Nature Methods 2, 677-684, 2005.
- Okabe, S., Urushido, T., Konno, D., Okado, H., and K. Sobue. Rapid redistribution of the postsynaptic density protein PSD-Zip45 (Homer 1c) and its differential regulation by NMDA receptors and calcium channels. Journal of Neuroscience, 21,9561-9571, 2001.
- Kuriu, T., Inoue, A., Bito, H., Sobue, K., and S. Okabe Differential control of postsynaptic density scaffolds via actin-dependent and independent mechanisms. Journal of Neuroscience 26, 7693-7706, 2006.
3. Astrocytic contacts promote synaptogenesis
Technology of single synapse visualization can be applied to the analysis of interaction between synapses and surrounding non-synaptic components. Major structural components present in the vicinity of synapses are processes of glial cells. Previous studies have shown that diffusible factors released from astroglia (a type of glial cells most intimately associated with synaptic structure) facilitate formation and maturation of synapses. Electron microscopic analysis confirmed the presence of direct contacts between synapses and astroglial processes. It has not yet been clarified if there is any local regulation on the development and maturation of single synapses by the direct contact of astroglial processes to synapses. In hippocampal slice culture preparations, contact events between astrocytic processes and dendritic spines were visualized by introducing two different chromophores into neurons and nearby astrocytes. By analyzing the relationship between astrocytic contacts and subsequent stabilization and maturation of spines, we found that local astrocytic contacts promote spine stabilization and structural maturation. Single astrocytes generate exclusive domains against the other astrocytes and contact with thousands of synapses within their own domains. Therefore astrocytes have a potential to either regulate single synapses locally or influence thousands of synapses within their domains at once. Molecules involved in local signaling from astrocytes to spines have not yet identified and future analyses should be necessary to clarify the relative contribution of diffusible factors and contact-mediated signaling in astrocyte-dependent facilitation of synapse development.
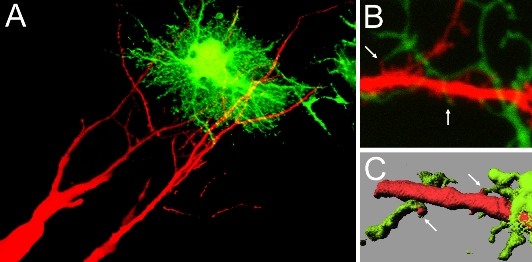 |
| III-5 Contact between dendritic spines and astrocytic processes. In A, hippocampal pyramidal neurons were filled with red fluorescent dye and astrocytes were infected with recombinant adenoviruses for GFP expression. Contact sites can be identified. In B, two-photon imaging revealed individual contact sites between spines (red) and astrocytic processes (greed). C shows an image after surface rendering, which facilitates visualization of contact sites between two cell types (arrows). |
- Nishida, H. and S. Okabe Direct astrocytic contacts regulate local maturation of dendritic spines. Journal of Neuroscience 27, 331-340, 2007.
4. Diversity in synapse formation
Excitatory synapses between pyramidal neurons in the hippocampu and the neocortex have been the default model of synapse formation in the CNS. However, the principles of synapse formation identified in one synapse subtype may not be applicable to other synapse subtypes. A clear example of unique principles in synapse formation was found in the case of excitatory synapses on dendrites of cortical interneurons (which are inhibitory neurons and use GABA as a main neurotransmitter). Although excitatory synapses on interneurons share common properties with those on pyramidal neurons, the strategy taken by interneurons was found to be quite unique by our imaging analyses. Namely, we found retrograde transport of synaptic structure along dendritic protrusions in immature interneurons. Translocating synapses gradually reach the main dendritic shafts and stop their movement. After maturation, dendritic protrusions are also eliminated and a majority of excitatory synapses remains on the surface of dendritic shafts. Interestingly, synapse translocation was driven by dynein motor proteins, which move along the rails of microtubules. This dynein-dependent motility is regulated by dynein-interacting molecule Lis1. Synapse mobility based on microtubule-dependent motor molecules is a novel finding and may contribute to clarification of cytoskeletal contribution in synapse formation and maturation.
A second example of unique strategies taken by specific neurons in synapse formation was found in the cerebellum. Detailed analyses of postnatal synapse development between cerebellar Purkinje cells and granule cell axons (parallel fibers) led to the discovery of small axonal protrusions at the contact sites with Purkinje cell dendrites. These axonal protrusions of granule cells wrap dendritic spines of Purkinje cells and facilitate synapse maturation. Formation of axonal protrusion was mediated by signaling pathway triggered by a soluble factor cbln1. This finding revealed sequential interplay between dendrites and axons plays an essential role in synapse maturation.
Even in the case of excitatory synapses in pyramidal neurons, which have been studied extensively, new principles and new regulatory molecules could be discovered by the progress of our understanding in nervous system development. Microtubule-associated protein DCLK1 shows very strong activity of facilitating microtubule assembly and stabilization. Interestingly, DCLK1 is preferentially localized at the tips of growing dendrites. This unique localization of DCLK1 helps facilitation of microtubule assembly at the tips of dendrites. On the other hand, DCLK1 shows suppressive roles in spine growth, assembly of PSD molecules, and postsynaptic receptor functions. Namely, DCLK1 controls dendritic growth positively and synapse maturation negatively at the tips of growing dendrites. The dual roles of DCLK1 may be critical in local control of dendritic functions during development.
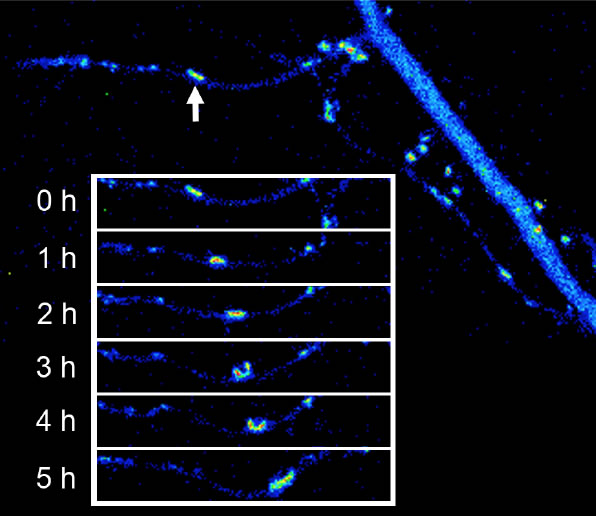 |
| III-6 PSD-95-GFP clusters translocating in a retrograde fashion along dendritic protrusions. These long protrusions are unique structure present in inhibitory neuron dendrites and are eliminated after maturation of inhibitory neurons. Synapses eventually reach dendritic shafts and become stationary. |
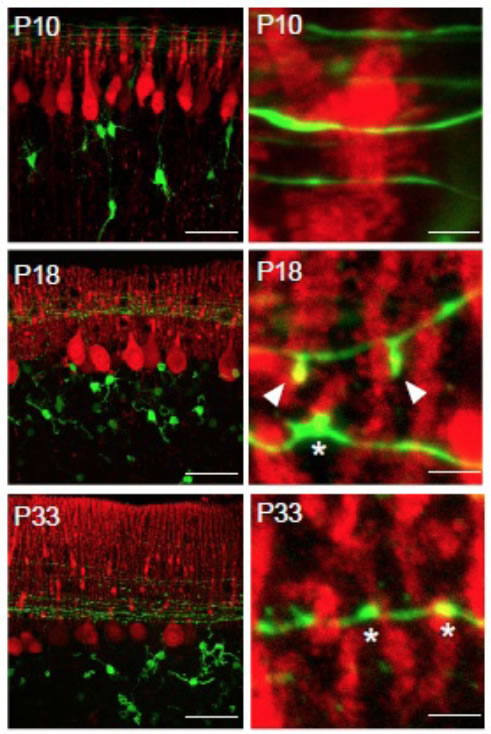 |
| III-7 Developmental changes in the morphology of synapses between parallel fibers (GFP signal, green) and Purkinje cell dendrites (anti-calbindin staining, red). At postnatal day 18, unique protrusive structures (arrowheads) can be identified, which contact with Purkinje cell spines. |
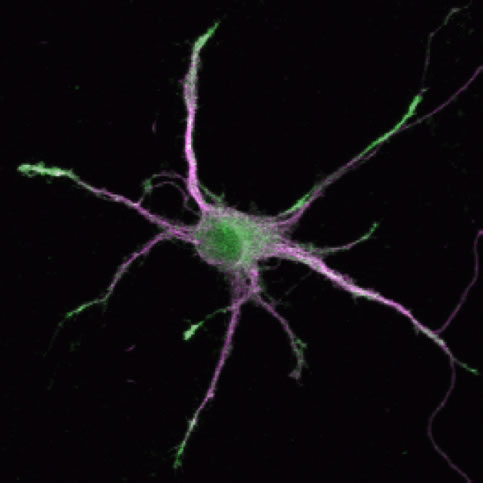 |
| III-8 DCLK1 is localized at the growing tips of dendrites and facilitate process growth. On the other hand, DCLK1 suppresses maturation of synapses locally and keep dynamics of distal dendrites. Green; anti-DCLK staining, Magenta; distribution of a dendritic marker MAP2. |
- Kawabata, I., Kashiwagi, Y., Obashi, K., Ohkura, M., Nakai, J., Wynshaw-Boris, A., Yanagawa, Y., and S. Okabe LIS1-dependent retrograde translocation of excitatory synapses in developing interneuron dendrites. Nature Communications 3, 722, 2012.74.
- Ito-Ishida, A., Miyazaki, T., Miura, E., Matsuda, K., Watanabe, M., Yuzaki, M and S. Okabe Presynaptically released Cbln1 induces dynamic axonal structural changes by interacting with GluD2 during cerebellar synapse formation. Neuron 76, 549-564, 2012.
- Shin, E., Kashiwagi, Y., Kuriu, T., Iwasaki, H., Tanaka, T., Koizumi, H., Gleeson, J. G. and S. Okabe Doublecortin-like kinase enhances dendritic remodeling and negatively regulates synapse maturation. Nature Communications 4, 1440, 2013.
IV Application of synapse imaging in studies of mental disorders
Technology of synapse imaging is now expanded to the field of in vivo synapse analyses. This was enabled by introduction of two-photon excitation laser scanning microscopy in the analyses of neuronal morphology and function in intact brain tissues. With adequate surgical techniques and cranial window preparations, the same cortical regions can be imaged repetitively and time-lapse images of dendrites and synapses in vivo can be recorded with time scales of minutes to months. By applying this imagine technology to the analyses of model mice of mental disorders, neural circuit-level dysfunctions in vivo may be identified. Autism spectrum disorders (ASDs) are early-onset metal disorders characterized by deficits in social behaviors, restricted interest, and repetitive behaviors. Recent genetic studies revealed strong link to genes encoding synaptic cell adhesion molecules and scaffolding proteins, suggesting functional changes of synapses in ASD patients. We analyzed three mouse models of ASDs with distinct genetic backgrounds and identified common phenotypes in synapse dynamics. Both synapse formation and elimination are enhanced in cortical pyramidal neurons during the early postnatal period. If future studies confirm enhancement of synapse turnover as a core feature of neural circuit in mouse models of ASDs, this finding will greatly contribute our understanding in pathophysiology of ASDs.
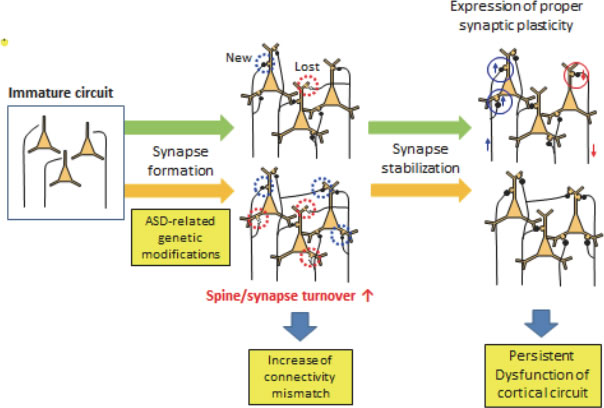 |
| IV-9 Proposed neural circuit alterations present in mouse models of ASDs. ASD-related genetic modifications will alter the speed of synapse turnover, which may increase the mismatches between pre- and postsynaptic components. These mismatches affect proper functions of the cortical neural circuits, which may be fixed after suppression of synapse dynamics in adult stage. |
- Isshiki, M., Tanaka, S., Kuriu, T., Tabuchi, K., Takumi, T. and S. Okabe
Enhanced synapse remodelling as a common phenotype in mouse models of autism.
Nature Communications 5, 4742, 2014. doi: 10.1038/ncomms5742.
V Future directions in the research of synapse cell biology
Cell biology of synapses has made significant progress after introduction of imaging technology, which enabled analyses of single synaptic structure and molecular assembly within the synaptic cytoplasm. I placed emphasis on the dynamics of PSD scaffolding proteins, which are the major interest of our research group, but there has also been rapid progress in our understanding of the behavior of synaptic vesicles in the presynaptic cytoplasm and the mobility and insertion of neurotransmitter receptors in the postsynaptic membrane. Based on these accumulating data on rapid structural remodeling and protein dynamics in synapses, future perspectives on the problem in the field of synapse cell biology will be discussed.
1. Building a quantitative model of synaptic molecular assembly
The molecular repoirtre in synapse architecture has been revealed by proteomic analysis of the biochemically purified synaptosomal fraction. Furthermore, quantitative analyses of proteins localized at synapses have reported absolute content of molecules per single synapses. Data on the molecular contents, together with the exchange rate of individual molecules, will facilitate our attempts to build realistic models on the molecular assembly at synapses. Electrophysiological analyses of synapses suggested that various functional alterations in synapses, such as long-term potentiation and depression, have relatively narrow dynamic range, which is in the order of 150-300% of the baseline value. Proposing molecular mechanisms that enable precise regulation of synaptic function with this dynamic range is challenging. Models should predict functional changes with high precision and accuracy. Functional changes associated with overexpression or knockdown of specific proteins should also be explained by using the same models. General principles on macromolecular assembly extracted from these models on synapses should be useful in other attempts to quantify the dynamic behavior of intracellular molecular assemblies in a variety of cells and organisms.
2. Behavior of synapses in vivo
Until recently, research on synapse biology was mainly based on analyses of dissociated neurons or tissue slices maintained in vitro. Information on synapse dynamics in living animals was not widely available. An important next step is to answer questions associated with functional and structural change of synapses during maturation of neural networks in situ. New optical techniques, especially two-photon excitation of GFP molecules expressed in neurons, are currently successfully applied to visualize individual synaptic structures in the superficial layer of the neocortex. We can expect that further development of imaging technologies and genetic manipulations will extend the limitation of imaging depth and the target brain structure. Future development of optogenetic technologies manipulating synaptic activity and neuronal firing in situ will also open the way to directly correlate synaptic functions and diverse behavior of animals.

